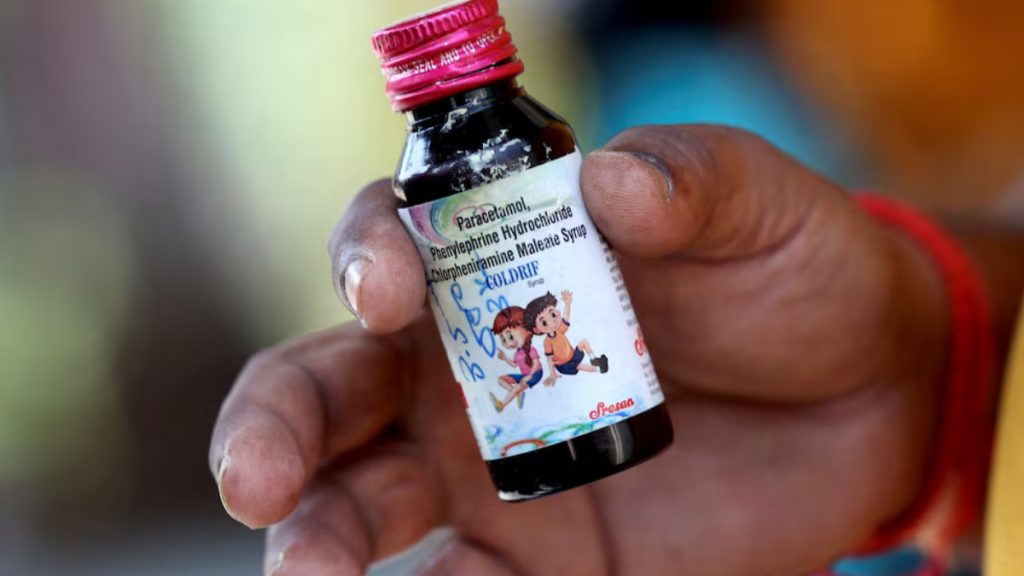
The tragic deaths of at least 20 children in Madhya Pradesh’s Chhindwara district have cast a harsh spotlight on the dangers lurking within India’s pharmaceutical supply chain. These children succumbed to acute kidney failure after ingesting Coldrif cough syrup, which was found to contain 48.6% diethylene glycol—a toxic industrial solvent used in antifreeze and brake fluid. This concentration exceeded permissible safety limits by nearly 500 times, turning a common remedy into a lethal poison. The contamination has not only devastated families but also raised urgent questions about the oversight and accountability of drug manufacturing practices.
Produced by Sreesan Pharma, a Tamil Nadu-based company, the Coldrif syrup was swiftly banned across Madhya Pradesh following the discovery. The ban, issued on October 5, was accompanied by the arrest of several company executives, signaling a reactive but necessary crackdown. Despite these measures, five more children remain hospitalized, underscoring the lingering threat and the need for immediate medical intervention and public health support. The incident has triggered widespread alarm, with authorities scrambling to trace the distribution network and recall any remaining stock from pharmacies and clinics.
This case is not an isolated one. Similar instances of diethylene glycol contamination have surfaced in neighboring states such as Rajasthan and Telangana, suggesting systemic flaws in quality control and regulatory enforcement. The recurrence of such tragedies points to a troubling pattern: inadequate testing, poor manufacturing standards, and insufficient monitoring by drug control agencies. While India is a global pharmaceutical powerhouse, these lapses reveal a dangerous underbelly where profit can sometimes eclipse safety, especially in the production of low-cost medicines for vulnerable populations.
The Coldrif incident serves as a grim reminder of the human cost of regulatory negligence. It calls for a comprehensive overhaul of India’s drug safety protocols, including stricter licensing procedures, routine audits, and harsher penalties for violations. Public trust in the healthcare system hinges on the assurance that medicines are safe and effective. Without robust reforms and transparent accountability, such tragedies risk becoming recurring headlines rather than rare exceptions. The lives lost in Chhindwara must not be in vain—they must catalyze lasting change.